
Products
Professional Design Butterfly Trainer - 3M Pre-Wire Disposable Neutral Electrode – Quanding
Professional Design Butterfly Trainer - 3M Pre-Wire Disposable Neutral Electrode – Quanding Detail:
Product Specification
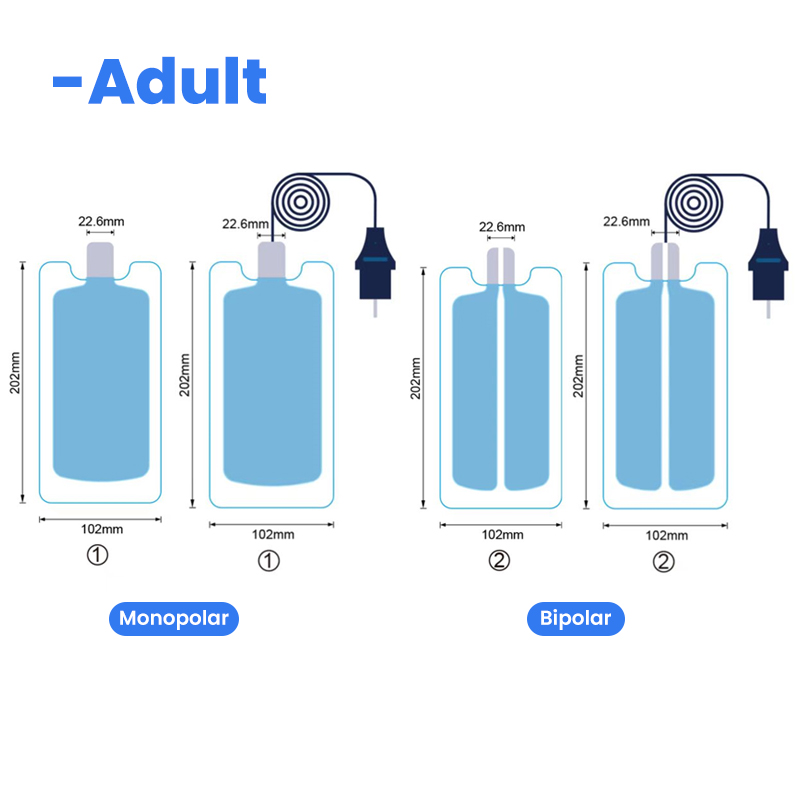
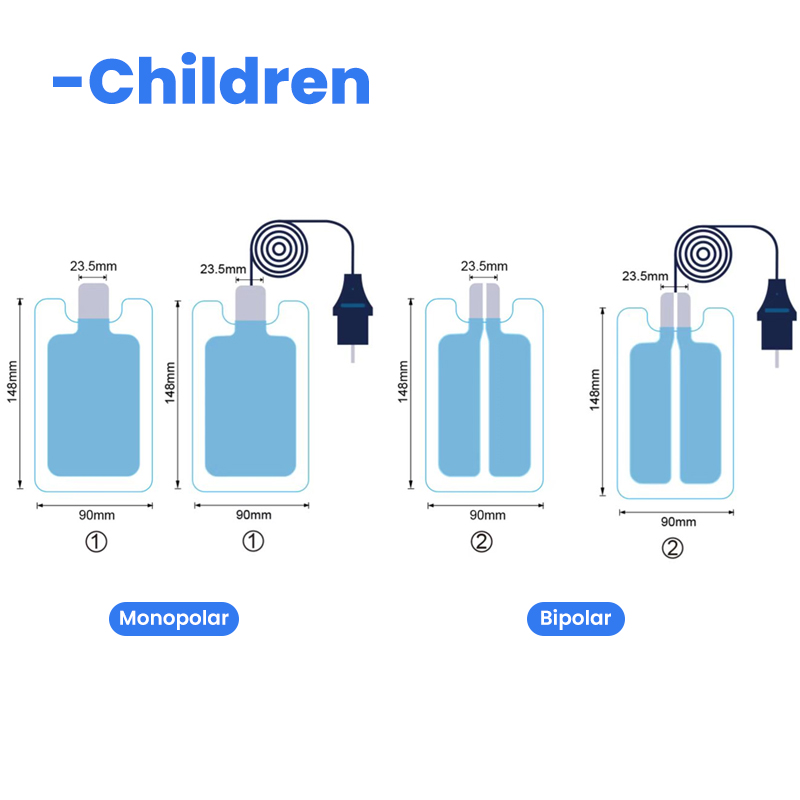
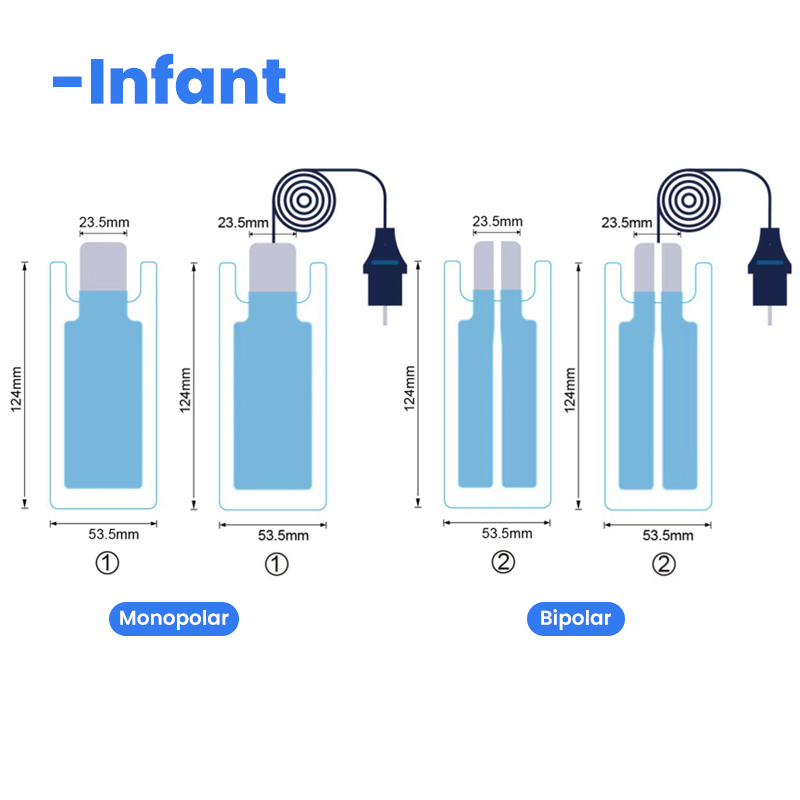
| For Adult | For Child | Infant |
| QD202B-A | QD202B-P | QD202B-1 |
| Bipolar /Split | Monopolar/single | Bipolar/Monopolar |
| 102*202mm | 90*148mm | 53.5*124mm |
| Packaging | 1pc/foil bag
350pcs/carton |
|
| Carton Size | 45*45*28CM
GW: 15.4kg |
|
| Certification | ISO13485,CE | |
|
Storage
|
The product must be stored in its original package at the environmental conditions (temperature and relative humidity) specified on the pouch’s label. Putting external heavy weights on the package could damage the product. | |
Product Instructions.
1. Select a well convex area (muscle) next to the area to be operated but at least 20 cm far from it, possibly on the forearm, on the thigh or on a whip. Eventually move the plate in order that the longer side is perpendicular to the area of the operation (see the picture). Avoid that the plate is superimposed.
2.NB Current should flow in parallel to the body surface and never pass through the thorax. The patient must be placed on a dry and perfectly isolated surface. The patient must be kept isolated from conductive parts and the operating bed must be earthed. Use dry gauzes to avoid that different areas of the body come into contact with each other.
3. Do not place the plate on wounds and scars, near bone projections, very adipose tissues, close to metallic prosthesis or to ECG electrodes
or on areas, where liquids can flow.
4.Do not place the plate on areas, where it can be subjected to pressure or under patient’s weight.
5.Shave the selected area, clean carefully and skim in order to remove eventual remaining of cream or cosmetics. Dry the selected area.
6.NB Do not use inflammables during the patient’s preparation. Do not use alcoholic substances or benzoine tincture.
7.Eventual shave remaining or hair can cause burns.
Application
- After controlling the packing for damages, check the plates and gel. Do not use the product in the case of damages or visible defects. Instead, return it to FIAB.
- Only open package immediately before use.
- Take off the protection film and place the plate on the prepared area; place it starting from a side and continue with same pressing until the opposite side.
- Make sure that the entire surface of the plate is in contact with the patient’s skin.
- Avoid excessive contact between fingers and adhesive. Do not touch the gel . NB There must not be air bubbles or parts of the adhesive support not perfectly close to the patient.
- Connect the plate to the high-frequency generator using the connectorcable . For neutral plate without connection cable: lift the connector lever and plug in the tongue.Make sure to plug in the tongue until the end, then close the lever. Then connect the generator to the pencil and the eventual foot control.
Warning
1. This is a disposable product – do not re-use.
2. The product is supplied not sterile – do not sterilize.
3. Do not re-position or cut the plate, do not add gel.
4. Do not use the product in case:
5. The packaging is not complete
6.There are evident damages on the plate or on the connection cable, Gel is not homogeneous or is dry.
7.Do not submit a patient with implanted pacemaker to electrosurgical current without first consulting a cardiologist.
8. The devices are to be used by trained healthcare professionals in electrosurgical procedures.
9. Do not use paediatric plates for high-power procedures (such as trans-urethral resection – TUR).
Product detail pictures:


Related Product Guide:
To constantly improve the management system by virtue of the rule of "sincerely, good faith and quality are the base of enterprise development", we widely absorb the essence of related products internationally, and constantly develop new products to meet the demands of customers for Professional Design Butterfly Trainer - 3M Pre-Wire Disposable Neutral Electrode – Quanding , The product will supply to all over the world, such as: Suriname, Belgium, Uruguay, After years of development, we have formed strong ability in new product development and strict quality control system to ensure excellent quality and service. With the support of many long term cooperated customers, our products are welcomed all over the world.
With a positive attitude of "regard the market, regard the custom, regard the science", the company works actively to do research and development. Hope we have a future business relationships and achieving mutual success.











